How do THE BAD GUYS (detergens) do it?
Detergents encapsulate tiny particles and drops of greasy dirt, to keep them floating in the surrounding aqueous environment, which is just a fancier wording of the water you use to wash the dirty stuff in. The encapsulating detergents being nothing more than the soap, when you wash your hands, the shampoo, when you wash your hair, and the washing powder, when you do your laundry.
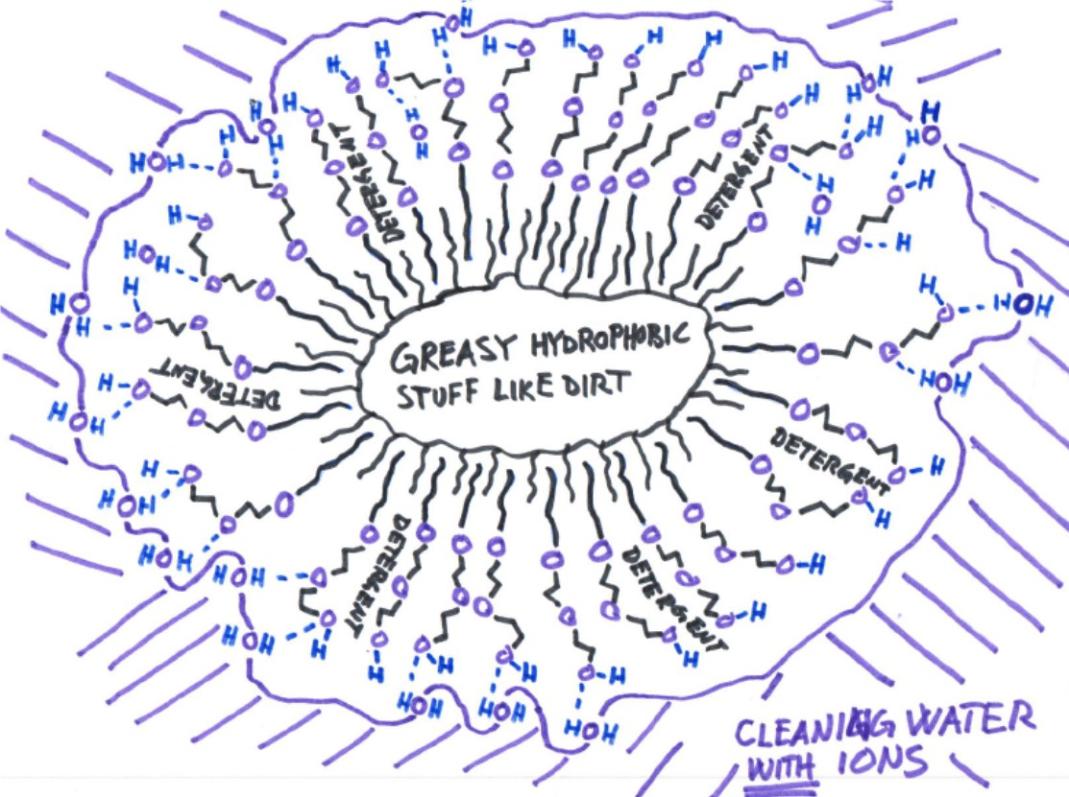
The hydrophopbic (i.e. greasy) end of the detergent molecule sticks to the hydrophopic surface of the greasy dirt, in accordance with the rule of thumb “like dissolves like”. The other hydrophilic (water loving) end feels very comfortable to be close to the water molecules of the washing water, not the least because it may form hydrogen bonds with the water molecules thereof. A hydrogen bond is a pretty strong bond in which a hydrogen atom (H) is treasured by two different oxygen atoms, which very often originate from two different molecules. One may be a water molecule of the washing water and the other may be a detergent molecule. The two different molecules are thereby held close to each other by such a hydrogen bond.
Hence, by having two different ends, one hydrophobic and one hydrophilic, the detergent molecule acts as mediator between the water and the greasy dirt, whereby the latter is enabled to float around in the former with a minimum of overall discontent.
There's even more to it... You may also want to have a look at this site.
How does NICE and friendly ultrapure water dissolve/emulgate fat and greasy dirt?
Short answer - no one knows how, we just know that it works! It's now up to the scientific community to come up with a solid theory.
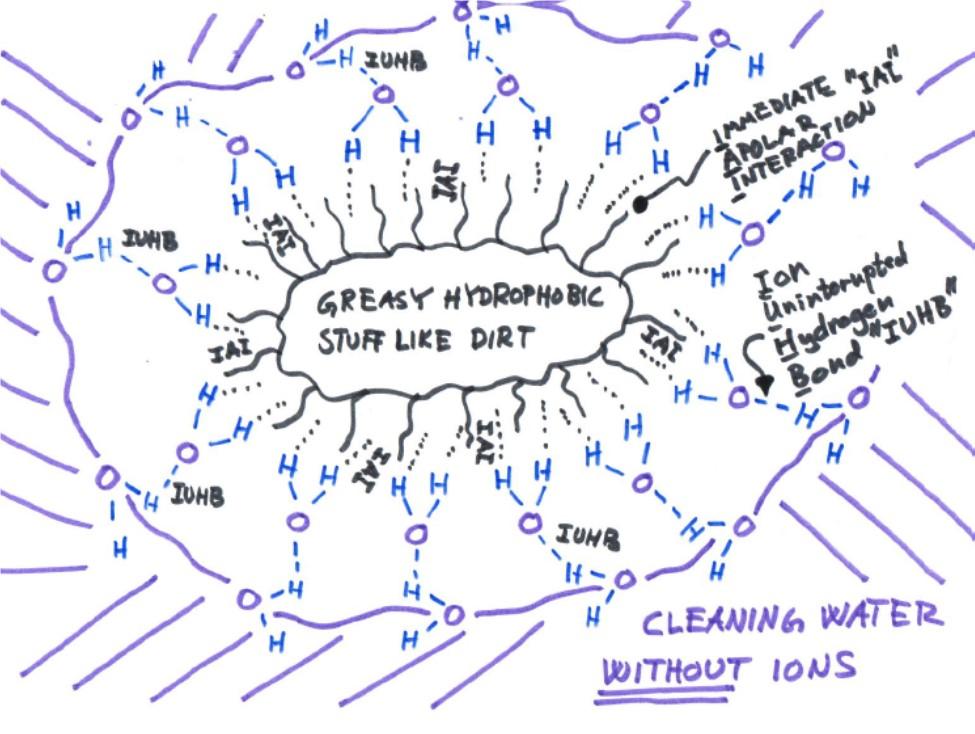
The current working hypothesis cartooned out below, which is much more elaborate than just calling the water "hungry" to dodge science, has been developed at Wepo’s labs by Wepo’s scientists and accounts both for the detrimental effect of dissolved ions, in particular cations (positively charged ions like Ca2+ and Na+), as well as the Immediate Apolar Interaction (IAI) between water and a hydrophobic surface, a necessity for water to carry this kind of dirt.
The IAI is basically a weak Van der Waals force between the hydrogens of the water and the hydrophobic surface of the greasy dirt, occurring independently of the presence or absence of ions. In the presence of cations like Ca2+, Mg2+ or Na+, however, the negative charge of the thereby exposed oxygen atoms of the IAI-participating water molecules will preferably coordinate to these ions instead of forming a hydrogen bond to the surrounding water. It is only when the water is free from such cations, like in Wepo’s ultrapure water, that hydrogen bonds necessary for interaction with the surrounding environment can form and thereby hold the greasy dirt in solution.
We don't claim to have nailed the final theory quite yet, as already said, we just know that it works – Wepo’s ultrapure water can accommodate greasy dirt long and stable enough to flush it away from whatever is being washed, at least as effectively as had a detergent been on board!
How do you know that it works - have you carried out experiments to prove it?
There is really no need for us to do that, but we have carried out a few just for confirmation. Ultrapure water has been used by others in applications were (greasy) dirt is removed from surfaces, like printed circuit bords, windows or floors, as well as from textiles. Their statements of success is surely evidence enough!
Considering that it dissolves/emulgates fat, is it then safe (or do you need to wear gloves)?
From a safety perspective, its still nothing more than just water! The IAI effect won't be applicable to cell-membranes. The lipid layer of such expose the hydrophilic part, not the hydrophobic one (like greasy dirt will). Ultrapure water is very selective, it can differ the fat of the dirt from the fat of your cell-membranes!
© Copyright Wepo AB